Here are the essential concepts you must grasp in order to answer the question correctly.
Atomic Structure
Atoms are the basic building blocks of matter, consisting of protons, neutrons, and electrons. The number of protons in the nucleus defines the element and its atomic number, while the arrangement of electrons determines the atom's chemical properties. Understanding atomic structure is essential for distinguishing between different elements.
Recommended video:
Elemental Properties
Different elements possess unique properties, such as atomic mass, electronegativity, and ionization energy. These properties arise from the differences in atomic structure and influence how elements interact with one another. For example, metals tend to lose electrons easily, while nonmetals gain them, leading to varied chemical behaviors.
Recommended video:
Chemical Properties Example 1
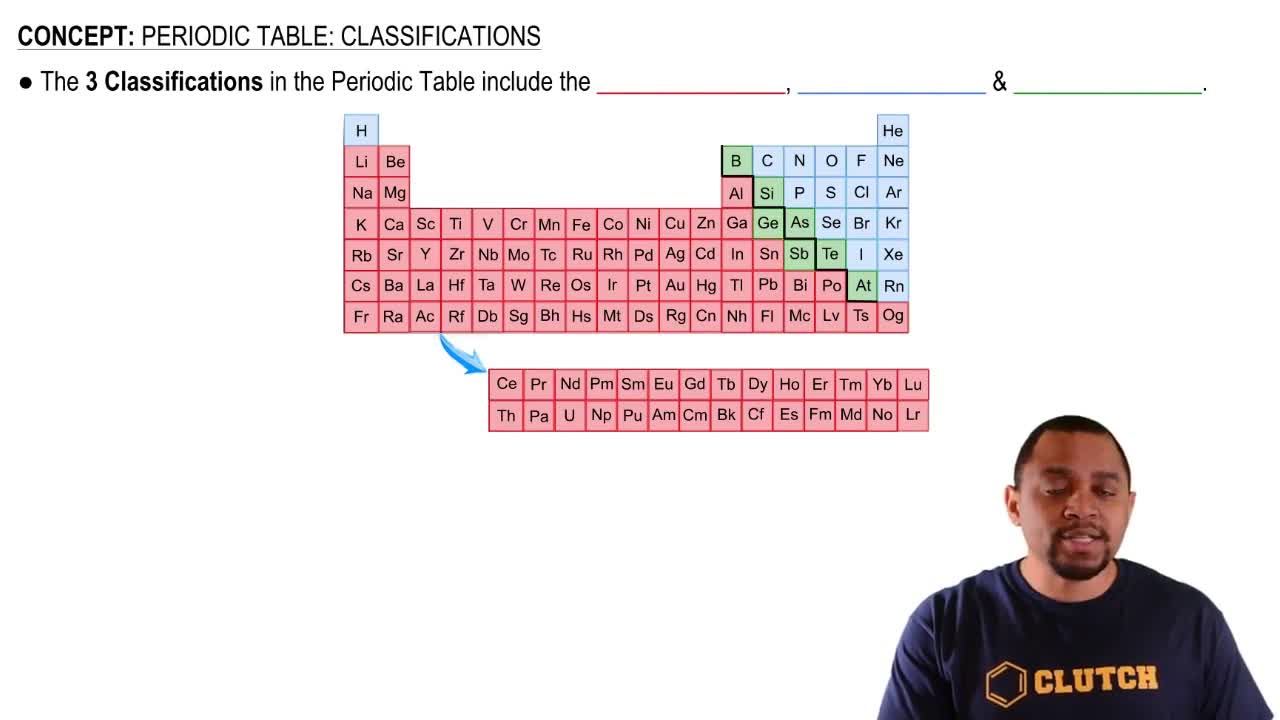
Periodic Table
The periodic table organizes elements based on their atomic number and properties, revealing trends and relationships among them. Elements in the same group exhibit similar chemical behaviors due to their valence electron configurations. This organization helps in predicting how different elements will react and interact with each other.
Recommended video:
Periodic Table: Classifications
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 0:53m
0:53m