Here are the essential concepts you must grasp in order to answer the question correctly.
pH Scale
The pH scale measures the acidity or alkalinity of a solution, ranging from 0 to 14. A pH of 7 is considered neutral, values below 7 indicate acidity, and values above 7 indicate alkalinity. Thus, the lower the pH value, the more acidic the solution.
Recommended video:
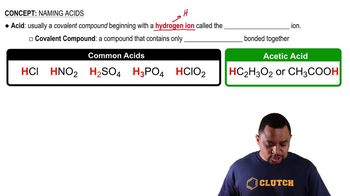
Acidity
Acidity refers to the concentration of hydrogen ions (H+) in a solution. Solutions with higher concentrations of H+ ions have lower pH values and are therefore more acidic. Understanding acidity is crucial for comparing the pH levels of different solutions.
Recommended video:
Comparative Analysis of pH
Comparative analysis of pH involves evaluating the pH values of different solutions to determine their relative acidity or alkalinity. In this case, by comparing the pH of solution X (9.0) and solution Y (7.0), one can conclude which solution is more acidic based on their respective pH values.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution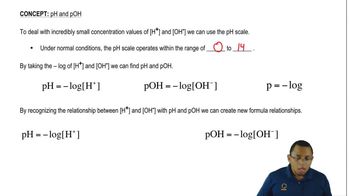


 1:31m
1:31m