Here are the essential concepts you must grasp in order to answer the question correctly.
Partial Pressure
Partial pressure refers to the pressure exerted by a single type of gas in a mixture of gases. In this scenario, the balloon contains helium and neon gases, each contributing to the total pressure. Understanding partial pressure is crucial for predicting how changes in gas composition or conditions affect the overall behavior of the gas mixture.
Recommended video:
Dalton's Law: Partial Pressure (Simplified) Concept 2
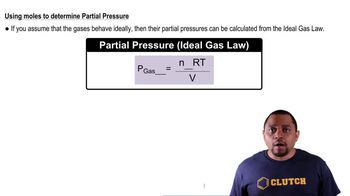
Ideal Gas Law
The Ideal Gas Law (PV=nRT) relates the pressure (P), volume (V), temperature (T), and number of moles (n) of a gas. In this question, the doubling of the Kelvin temperature while maintaining pressure allows us to infer changes in volume. This law is fundamental for understanding how gases behave under varying conditions.
Recommended video:
Gas Leakage and Volume Change
When gas leaks from a balloon, the number of gas particles decreases, which can lead to a reduction in volume if the temperature and pressure remain constant. In this case, half of the gas atoms leaking out will affect the balloon's volume, and understanding this concept is essential for predicting the final state of the balloon after the specified changes.
Recommended video:
Physical & Chemical Changes