Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They help visualize the arrangement of electrons and the connectivity of atoms, which is crucial for understanding molecular geometry and reactivity. In the case of CH₄ (methane) and H₂O (water), their Lewis structures illustrate how the atoms are bonded and the distribution of electron pairs.
Recommended video:
Lewis Dot Structures: Ions (Simplified) Concept 1
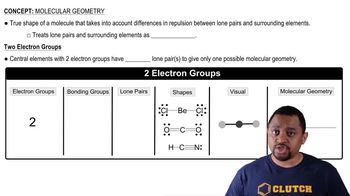
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of bonding pairs and lone pairs of electrons around the central atom, which influence the shape of the molecule. For CH₄, the tetrahedral geometry results from four bonding pairs, while H₂O has a bent shape due to two bonding pairs and two lone pairs, affecting their bond angles.
Recommended video:
Molecular Geometry (Simplified) Concept 1
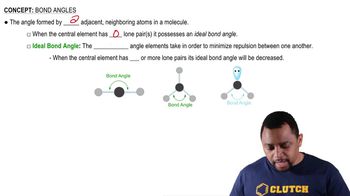
Bond Angles
Bond angles are the angles formed between adjacent bonds in a molecule, which are influenced by the repulsion between electron pairs. In CH₄, the bond angles are approximately 109.5 degrees due to its tetrahedral shape, while in H₂O, the bond angle is about 104.5 degrees, reflecting the repulsion from the lone pairs. Despite the difference in shapes, both molecules exhibit similar bond angles due to the tetrahedral arrangement of electron pairs.
Recommended video:
Bond Angles (Simplified) Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 1:24m
1:24m