Here are the essential concepts you must grasp in order to answer the question correctly.
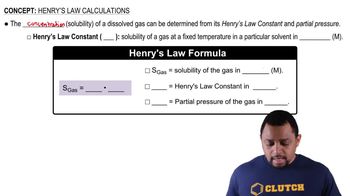
Henry's Law
Henry's Law states that the amount of gas that dissolves in a liquid is directly proportional to the partial pressure of that gas above the liquid. In the context of hyperbaric chambers, increasing the pressure allows more oxygen and nitrogen to dissolve in the blood, which is crucial for treating conditions like decompression sickness and carbon monoxide poisoning.
Recommended video:
Henry's Law Calculations Concept 1
Decompression Sickness
Decompression sickness, also known as 'the bends,' occurs when nitrogen dissolved in the blood forms bubbles as pressure decreases during ascent from deep water. Hyperbaric treatment helps by increasing the pressure, allowing excess nitrogen to dissolve back into the blood, thus preventing bubble formation and alleviating symptoms.
Carbon Monoxide Poisoning
Carbon monoxide poisoning occurs when CO binds to hemoglobin in red blood cells more effectively than oxygen, reducing oxygen transport. Hyperbaric oxygen therapy increases the amount of dissolved oxygen in the blood, displacing carbon monoxide from hemoglobin and facilitating its elimination from the body, thereby improving oxygen delivery to tissues.
Recommended video:
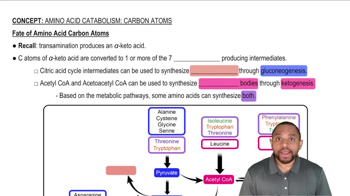
Amino Acid Catabolism: Carbon Atoms Concept 2