Here are the essential concepts you must grasp in order to answer the question correctly.
Hemiacetals and Hemiketals
Hemiacetals and hemiketals are organic compounds formed when an alcohol reacts with an aldehyde or a ketone, respectively. A hemiacetal consists of a carbon atom bonded to an -OH group, an -OR group (from the alcohol), a hydrogen atom, and an alkyl or aryl group. In contrast, a hemiketal has a similar structure but is derived from a ketone, featuring two alkyl or aryl groups instead of one.
Recommended video:
Cyclic Hemiacetals Concept 1
Reaction Mechanism
The formation of hemiacetals and hemiketals involves a nucleophilic addition reaction, where the nucleophile (alcohol) attacks the electrophilic carbon of the carbonyl group (aldehyde or ketone). This reaction typically occurs in two steps: first, the nucleophile adds to the carbonyl carbon, forming a tetrahedral intermediate, followed by proton transfer and the loss of water to yield the hemiacetal or hemiketal.
Recommended video:
Alcohol Reactions: Dehydration Reactions Concept 1
Equilibrium and Stability
The formation of hemiacetals and hemiketals is an equilibrium process, meaning that the reaction can proceed in both directions. The stability of these compounds is influenced by factors such as steric hindrance and the nature of the substituents on the carbon atom. Understanding this equilibrium is crucial for predicting the predominant forms in a given reaction environment, especially in the presence of excess alcohol or water.
Recommended video:
The Equilibrium Constant Example 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution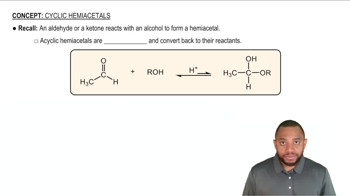



 1:49m
1:49m