Here are the essential concepts you must grasp in order to answer the question correctly.
Atomic Number
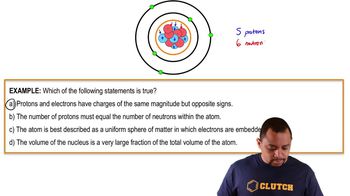
The atomic number is the number of protons found in the nucleus of an atom. It uniquely identifies an element and determines its position on the periodic table. For example, carbon has an atomic number of 6, meaning it has 6 protons. The atomic number is crucial for understanding the chemical properties of an element.
Recommended video:
Calculate Oxidation Numbers
Mass Number
The mass number is the total number of protons and neutrons in an atom's nucleus. It provides insight into the atom's overall mass and stability. For instance, a carbon atom with 6 protons and 6 neutrons has a mass number of 12. The mass number is essential for distinguishing between isotopes of an element, which have the same atomic number but different mass numbers.
Recommended video:
Nucleus Composition
The nucleus of an atom is composed of protons and neutrons, collectively known as nucleons. The number of particles in the nucleus can be determined by the mass number, as it reflects the total count of these nucleons. Understanding the composition of the nucleus is fundamental in nuclear physics and chemistry, as it influences the atom's behavior in reactions and its stability.
Recommended video:
The Atom (Simplified) Example 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:25m
1:25m