Here are the essential concepts you must grasp in order to answer the question correctly.
Dalton's Law of Partial Pressures
Dalton's Law states that in a mixture of gases, the total pressure exerted is equal to the sum of the partial pressures of each individual gas. This principle allows us to calculate the pressure of one gas in a mixture if we know the total pressure and the partial pressures of the other gases present.
Recommended video:
Dalton's Law: Partial Pressure (Simplified) Concept 3
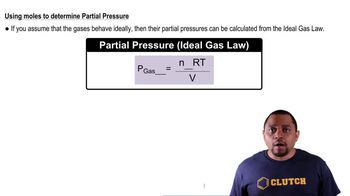
Partial Pressure
Partial pressure refers to the pressure that a single gas in a mixture would exert if it occupied the entire volume alone at the same temperature. It is a crucial concept for understanding gas behavior in mixtures and is calculated using the formula: P_partial = P_total - P_other_gases.
Recommended video:
Dalton's Law: Partial Pressure (Simplified) Concept 2
Gas Laws
Gas laws describe the relationships between pressure, volume, temperature, and the number of moles of a gas. In this context, understanding how these variables interact helps in solving problems related to gas mixtures, particularly when applying the ideal gas law or Dalton's Law.
Recommended video:
Chemistry Gas Laws: Combined Gas Law
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 0:44m
0:44m