Here are the essential concepts you must grasp in order to answer the question correctly.
Rutherford's Gold-Foil Experiment
Rutherford's gold-foil experiment, conducted in 1909, involved firing alpha particles at a thin sheet of gold. Most particles passed through, but some were deflected at large angles, indicating that the atom is mostly empty space with a small, dense nucleus. This unexpected result challenged the existing plum pudding model of the atom and laid the groundwork for the nuclear model.
Recommended video:
Rutherford Gold Foil Oil Experiment
Nuclear Model of the Atom
The nuclear model of the atom, proposed by Rutherford, suggests that an atom consists of a small, positively charged nucleus surrounded by negatively charged electrons. This model replaced the earlier plum pudding model, emphasizing the concentration of mass and positive charge in the nucleus, which accounts for the deflection of alpha particles during the experiment.
Recommended video:
Molecular Models Example 1
Deflection of Alpha Particles
The deflection of alpha particles in Rutherford's experiment is crucial for understanding atomic structure. The degree of deflection depended on the proximity of the alpha particles to the nucleus; significant deflections indicated a strong positive charge concentrated in a small volume. This observation led to the conclusion that the nucleus is the core of the atom, influencing the development of modern atomic theory.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution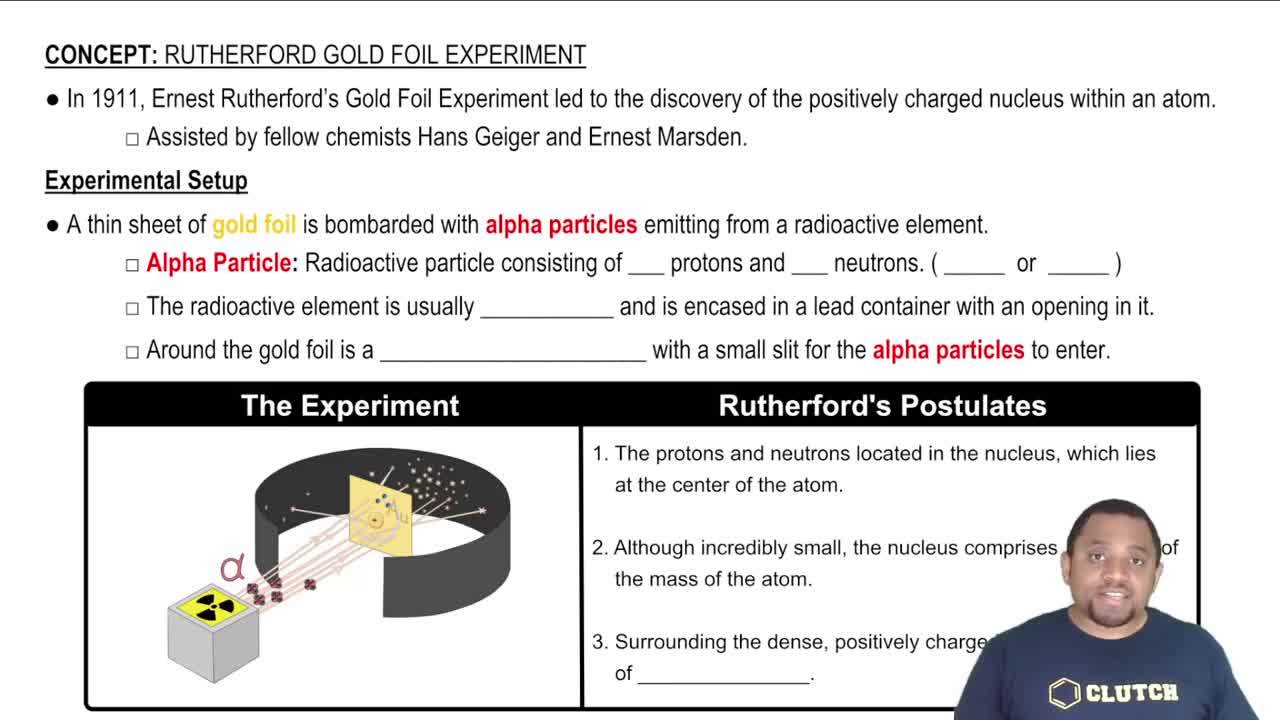



 3:27m
3:27m