Here are the essential concepts you must grasp in order to answer the question correctly.
Weak Acids
Weak acids, like acetic acid (CH₃CO₂H), do not fully dissociate in water. Instead, they establish an equilibrium between the undissociated acid and its ions. This partial ionization is crucial for understanding their behavior in solution, as it affects pH and the acid's reactivity.
Recommended video:
Acid and Base Strength Concept 4
Dissociation Equilibrium
When a weak acid dissolves in water, it reaches a state of dissociation equilibrium, represented by the equation CH₃CO₂H ⇌ CH₃CO₂⁻ + H⁺. This means that some molecules remain intact while others break apart into ions, influencing the concentration of hydrogen ions and thus the solution's acidity.
Recommended video:
The Equilibrium Constant Example 1
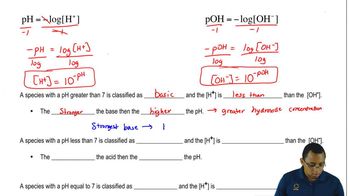
pH and Acidity
The pH scale measures the acidity of a solution, with lower values indicating higher acidity. When a weak acid like CH₃CO₂H is dissolved in water, it contributes hydrogen ions (H⁺) to the solution, lowering the pH. Understanding this relationship is essential for predicting the behavior of weak acids in various chemical contexts.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution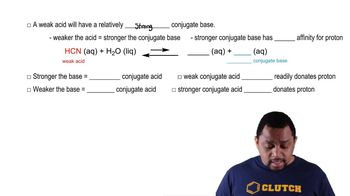


 3:17m
3:17m