Here are the essential concepts you must grasp in order to answer the question correctly.
Acids and Bases
Acids are substances that can donate protons (H+) in a solution, while bases are substances that can accept protons or donate hydroxide ions (OH-). This fundamental distinction is crucial for classifying compounds and understanding their chemical behavior in reactions.
Recommended video:
Arrhenius Acids & Bases Concept 1
Hydroxides
Hydroxides are compounds that contain the hydroxide ion (OH-). They are typically associated with bases, as they can increase the concentration of OH- in a solution, thereby raising the pH and making the solution more alkaline.
Recommended video:
Barium Hydroxide
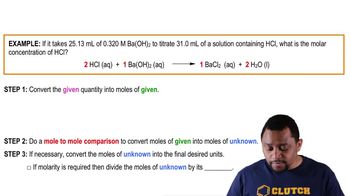
Barium hydroxide (Ba(OH)2) is a strong base formed from barium, a metal, and hydroxide ions. It is characterized by its ability to dissociate completely in water, releasing hydroxide ions, which is a key property of bases.
Recommended video:
Strong Acid Strong Base Titrations (Simplified) Example 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 0:40m
0:40m