Here are the essential concepts you must grasp in order to answer the question correctly.
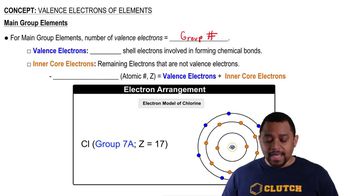
Group Number
The group number in the periodic table indicates the number of valence electrons in the outermost shell of an element. Chlorine is located in Group 17 (or VIIA), which means it has seven valence electrons. This classification helps predict the element's chemical behavior and reactivity.
Recommended video:
Calculate Oxidation Numbers
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's electron shells and subshells. For chlorine, the electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁵, indicating how electrons are arranged in the various energy levels. This configuration is crucial for understanding the element's bonding and reactivity.
Recommended video:
The Electron Configuration: Condensed
Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom that are involved in chemical bonding. For chlorine, the valence-shell configuration is 3s² 3p⁵, highlighting that it has five electrons in the p subshell and two in the s subshell. These electrons play a key role in forming bonds with other elements.
Recommended video:
Valence Electrons of Elements (Simplified) Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 0:54m
0:54m