Here are the essential concepts you must grasp in order to answer the question correctly.
Carbonyl Group
The carbonyl group is a functional group characterized by a carbon atom double-bonded to an oxygen atom (C=O). It is a key feature in various organic compounds, including aldehydes and ketones. The reactivity and properties of compounds containing carbonyl groups are largely influenced by the nature of the surrounding atoms or groups.
Recommended video:
Functional Groups with Carbonyls Example 3
Aldehydes
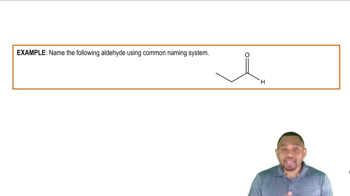
Aldehydes are organic compounds that contain a carbonyl group (C=O) at the end of a carbon chain, making them terminal functional groups. They are typically represented by the general formula RCHO, where R is a hydrocarbon group. Aldehydes are known for their distinctive odors and are commonly used in the production of various chemicals and fragrances.
Recommended video:
Naming Aldehydes Example 2
Ketones
Ketones are organic compounds that feature a carbonyl group (C=O) located within a carbon chain, making them internal functional groups. They are represented by the general formula RC(=O)R', where R and R' are hydrocarbon groups. Ketones are important in both industrial applications and biological processes, often serving as solvents or intermediates in chemical reactions.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution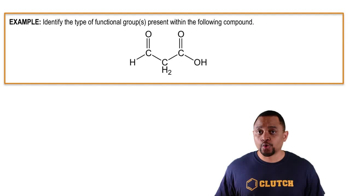

 2:38m
2:38m