Here are the essential concepts you must grasp in order to answer the question correctly.
Osmolarity
Osmolarity is a measure of the total concentration of solute particles in a solution. It is expressed in osmoles per liter (osmol/L) and takes into account all particles that contribute to the solution's osmotic pressure, including ions and molecules. For ionic compounds like NaOH, which dissociates into sodium (Na+) and hydroxide (OH-) ions, the osmolarity is calculated by multiplying the molarity by the number of particles produced upon dissociation.
Recommended video:
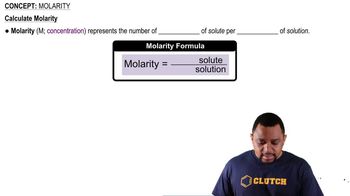
Molarity
Molarity is a way to express the concentration of a solution, defined as the number of moles of solute per liter of solution (mol/L). In the case of 0.30 M NaOH, this means there are 0.30 moles of NaOH in one liter of solution. Understanding molarity is essential for calculating osmolarity, especially for solutions that dissociate into multiple ions.
Recommended video:
Mass/Volume Percent Concentration
Mass/volume percent concentration (m/v) is a way to express the concentration of a solution as the mass of solute per 100 mL of solution. For example, a 3.0% (m/v) NaOH solution means there are 3 grams of NaOH in 100 mL of solution. This concept is important for converting to molarity and subsequently calculating osmolarity, especially when dealing with solutions that are not expressed in molarity.
Recommended video:
Percent Concentrations Concept 1
 Verified Solution
Verified Solution