Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolytes vs. Nonelectrolytes
Electrolytes are substances that dissociate into ions when dissolved in water, allowing the solution to conduct electricity. In contrast, nonelectrolytes do not dissociate into ions; they remain as intact molecules in solution. Understanding this distinction is crucial for determining the behavior of solutes like fructose in aqueous solutions.
Recommended video:
Electrolytes (Simplified) Concept 3
Dissociation in Aqueous Solutions
Dissociation refers to the process by which a compound separates into its constituent ions in a solvent, typically water. This process is essential for electrolytes, as it affects the solution's conductivity and chemical properties. For nonelectrolytes like fructose, dissociation does not occur, and the solute remains as whole molecules.
Recommended video:
Properties of Fructose
Fructose is a simple sugar (monosaccharide) that is classified as a nonelectrolyte. When dissolved in water, it does not produce ions but instead exists as individual fructose molecules. This characteristic influences its solubility, sweetness, and behavior in biological systems, making it important to recognize its molecular nature in aqueous solutions.
Recommended video:
Chemical Properties Example 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution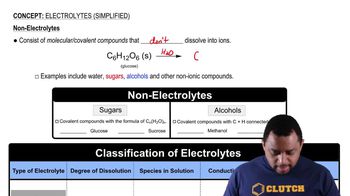



 2:38m
2:38m