Octet Rule
The octet rule is a chemical guideline that states atoms tend to bond in such a way that they each have eight electrons in their valence shell, achieving a stable electron configuration similar to that of noble gases. However, some molecules, like BeCl₂, do not adhere to this rule, as beryllium can form stable compounds with fewer than eight electrons, highlighting exceptions in chemical bonding.
Recommended video:
Ions and the Octet Rule Concept 1
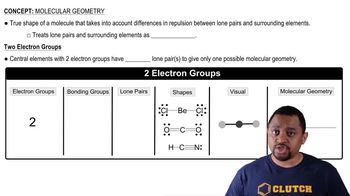
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is influenced by the number of bonds and lone pairs around the central atom, which can affect the molecule's reactivity and properties. Understanding molecular geometry is crucial for predicting how molecules will interact in chemical reactions and their physical characteristics.
Recommended video:
Molecular Geometry (Simplified) Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 0:52m
0:52m