Here are the essential concepts you must grasp in order to answer the question correctly.
Conjugate Acid-Base Theory
The conjugate acid-base theory, developed by Brønsted and Lowry, defines acids as proton donors and bases as proton acceptors. When a base accepts a proton (H⁺), it forms its conjugate acid. Understanding this relationship is crucial for predicting the behavior of chemical species in acid-base reactions.
Recommended video:
Acid and Base Strength Concept 3
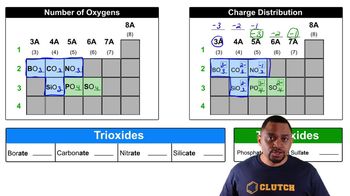
Carbonate Ion (CO₃²⁻)
The carbonate ion (CO₃²⁻) is a polyatomic ion consisting of one carbon atom covalently bonded to three oxygen atoms, carrying a -2 charge. It acts as a base in chemical reactions, capable of accepting protons to form its conjugate acid. Recognizing its structure and charge is essential for determining its conjugate acid.
Recommended video:
Conjugate Acid of Carbonate
The conjugate acid of the carbonate ion (CO₃²⁻) is bicarbonate (HCO₃⁻). When CO₃²⁻ accepts a proton, it transforms into HCO₃⁻, which can further act as an acid in subsequent reactions. Identifying the conjugate acid is vital for understanding the dynamics of acid-base equilibria involving carbonate species.
Recommended video:
Amino Acid Catabolism: Carbon Atoms Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution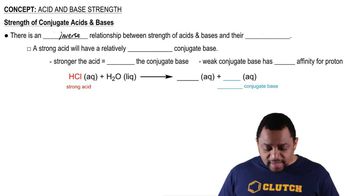


 0:40m
0:40m