Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility of Gases
Solubility refers to the ability of a substance (solute) to dissolve in a solvent. For gases, solubility is influenced by temperature, pressure, and the nature of the gas and solvent. Generally, the solubility of gases in liquids decreases as temperature increases, which is critical for understanding gas behavior in aquatic environments.
Recommended video:
Henry's Law
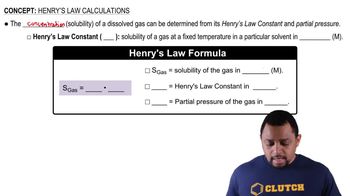
Henry's Law states that the amount of gas that dissolves in a liquid at a given temperature is directly proportional to the partial pressure of that gas above the liquid. This principle helps explain how changes in temperature and pressure affect gas solubility, particularly in solutions like water where gases like carbon dioxide and oxygen are involved.
Recommended video:
Henry's Law Calculations Concept 1
Thermal Energy and Molecular Motion
As temperature increases, the thermal energy of molecules also increases, leading to greater molecular motion. This increased motion can disrupt the interactions between gas molecules and the solvent, resulting in lower solubility of gases in liquids. Understanding this relationship is essential for predicting how temperature changes will affect gas solubility.
Recommended video:
Thermal Equilibrium (Simplified) Concept 1

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 4:38m
4:38m