Here are the essential concepts you must grasp in order to answer the question correctly.
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. They determine the properties and reactivity of organic compounds. Common functional groups include hydroxyl (-OH), carboxyl (-COOH), and amine (-NH2), each influencing the behavior of the compound in chemical reactions.
Recommended video:
Functional Group Priorities Concept 1
Hydrolysis
Hydrolysis is a chemical reaction in which water is used to break down a compound. In organic chemistry, it often involves the cleavage of bonds in larger molecules, resulting in the formation of smaller molecules. For example, esters can be hydrolyzed to produce an alcohol and a carboxylic acid, illustrating how water can facilitate the transformation of functional groups.
Recommended video:
Acidic Hydrolysis Concept 1
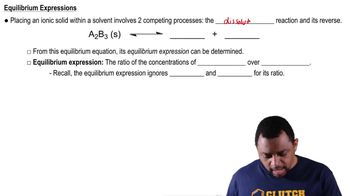
Product Structures
The product structures refer to the molecular configurations that result from a chemical reaction, such as hydrolysis. Understanding how to draw these structures involves knowing the original compound's functional groups and how they change during the reaction. This includes recognizing bond formations and breakages, which are essential for predicting the final products of the hydrolysis process.
Recommended video:
Solubility Product Constant (Ksp) Concept 2
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:12m
1:12m