Here are the essential concepts you must grasp in order to answer the question correctly.
Radioactive Decay
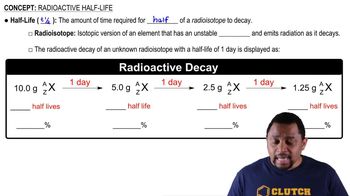
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. This decay occurs at a predictable rate, characterized by the half-life, which is the time required for half of the radioactive substance to decay. Understanding this concept is crucial for interpreting decay curves, as they visually represent the decrease in quantity of a radioactive isotope over time.
Recommended video:
Measuring Radioactivity Concept 1
Half-Life
Half-life is a specific measure of time that indicates how long it takes for half of a given quantity of a radioactive substance to decay. For iodine-131, the half-life is approximately 8 days. This concept is essential for calculating the remaining amount of a substance at any given time and is fundamental in fields such as nuclear medicine and radiometric dating.
Recommended video:
Radioactive Half-Life Concept 1
Decay Curve
A decay curve is a graphical representation that shows the decrease in quantity of a radioactive substance over time. The x-axis typically represents time (in days, for example), while the y-axis shows the remaining amount of the substance. Analyzing the decay curve allows one to determine the rate of decay and predict the amount of substance remaining at specific intervals, which is vital for solving problems related to radioactive materials.
Recommended video:

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 2:09m
2:09m