Here are the essential concepts you must grasp in order to answer the question correctly.
Conjugate Base
A conjugate base is the species that remains after an acid donates a proton (H⁺). In the context of Brønsted-Lowry theory, when an acid loses a proton, it transforms into its conjugate base, which can then potentially accept a proton in a chemical reaction.
Recommended video:
Acid and Base Strength Concept 3
Acid-Base Reaction
An acid-base reaction involves the transfer of protons between reactants. Acids are proton donors, while bases are proton acceptors. Understanding this interaction is crucial for identifying conjugate pairs, as each acid has a corresponding conjugate base formed after the proton transfer.
Recommended video:
Acid-Base Reactions Concept 1
Bicarbonate Ion (HCO₃⁻)
The bicarbonate ion (HCO₃⁻) is a weak acid that can donate a proton to form its conjugate base, the carbonate ion (CO₃²⁻). Recognizing the structure and behavior of bicarbonate is essential for determining its conjugate base and understanding its role in acid-base equilibria.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution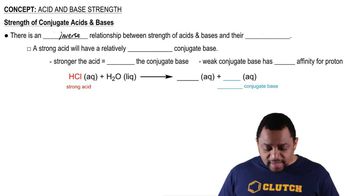



 0:40m
0:40m