Table of contents
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
20. Carbohydrates
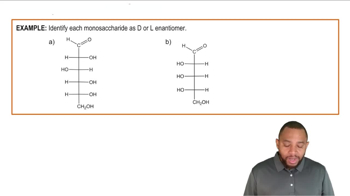
D vs L Enantiomers
Problem 6.17b
Textbook Question
Use the structure of d-galactose in Problem 6.15 to answer the following:
(b) Draw the Fischer projection of l-galactose.
 Verified step by step guidance
Verified step by step guidance1
Understand the structure of D-galactose: D-galactose is an aldohexose, which means it is a six-carbon sugar with an aldehyde group. In its Fischer projection, the hydroxyl groups (OH) are arranged in a specific pattern along the carbon chain.
Identify the stereochemistry of D-galactose: In the Fischer projection of D-galactose, the hydroxyl groups on the chiral centers (C2, C3, C4, and C5) are arranged as follows: C2 - OH on the right, C3 - OH on the left, C4 - OH on the right, C5 - OH on the right.
Convert D-galactose to L-galactose: To convert D-galactose to L-galactose, invert the configuration at every chiral center. This means that for L-galactose, the hydroxyl groups will be: C2 - OH on the left, C3 - OH on the right, C4 - OH on the left, C5 - OH on the left.
Draw the Fischer projection for L-galactose: Start with the aldehyde group at the top (C1), then draw the carbon chain vertically. Place the hydroxyl groups according to the L-galactose configuration determined in the previous step.
Verify the structure: Ensure that the drawn Fischer projection correctly represents L-galactose by checking that the hydroxyl groups are placed as determined and that the overall structure is consistent with the L-configuration.
Recommended similar problem, with video answer:
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Fischer Projection
The Fischer projection is a two-dimensional representation of a molecule that shows the configuration of its stereocenters. In this format, vertical lines represent bonds that go behind the plane of the page, while horizontal lines represent bonds that come out of the page. This method is particularly useful for visualizing carbohydrates and amino acids, allowing for easy identification of their stereochemistry.
Recommended video:
Guided course

Fischer Projections Example 1
D-Galactose vs. L-Galactose
D-galactose and L-galactose are stereoisomers, differing in the configuration around one or more of their chiral centers. The 'D' and 'L' designations refer to the orientation of the hydroxyl group on the penultimate carbon (the second to last carbon) in the Fischer projection. Understanding this distinction is crucial for accurately drawing the Fischer projection of L-galactose based on the structure of D-galactose.
Recommended video:
Guided course

D vs L Enantiomers Concept 1
Stereochemistry
Stereochemistry is the study of the spatial arrangement of atoms in molecules and how this affects their chemical properties and reactions. In carbohydrates like galactose, the specific arrangement of hydroxyl groups and hydrogen atoms around chiral centers determines the molecule's identity and function. A solid grasp of stereochemistry is essential for accurately representing and manipulating molecular structures in organic chemistry.
Recommended video:
Guided course

D vs L Enantiomers Concept 1

 1:3m
1:3mWatch next
Master D vs L Enantiomers Concept 1 with a bite sized video explanation from Jules Bruno
Start learning