Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Notation
Nuclear notation is a way of representing atomic nuclei using symbols that indicate the number of protons and neutrons. In this notation, the element symbol is preceded by two numbers: the atomic number (number of protons) as a subscript and the mass number (total number of protons and neutrons) as a superscript. This system allows for the identification of isotopes and helps in understanding nuclear reactions.
Recommended video:
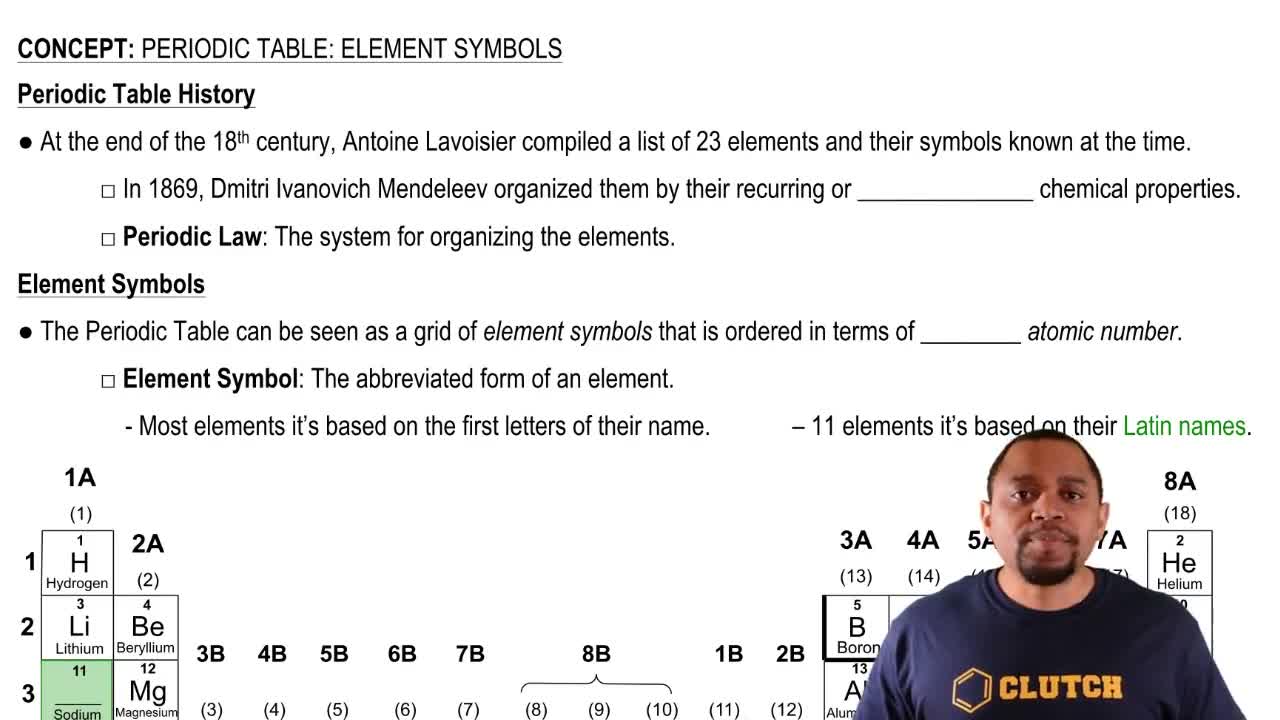
Element Symbol
An element symbol is a one- or two-letter abbreviation used to represent a chemical element on the periodic table. Each symbol is unique to an element and is derived from its English or Latin name. For example, 'X' could represent an unknown or generic element in a hypothetical context, while 'H' stands for hydrogen. Understanding element symbols is crucial for interpreting chemical equations and nuclear reactions.
Recommended video:
Periodic Table: Symbols Concept
Isotopes
Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons, resulting in different mass numbers. For instance, carbon-12 and carbon-14 are isotopes of carbon, with mass numbers of 12 and 14, respectively. Isotopes play significant roles in fields such as radiometric dating, nuclear medicine, and understanding nuclear stability.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:44m
1:44m