Here are the essential concepts you must grasp in order to answer the question correctly.
Acids and Bases
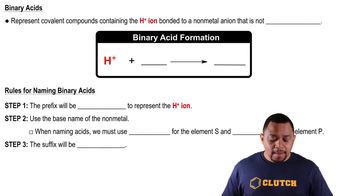
Acids are substances that can donate protons (H⁺ ions) in a solution, while bases are substances that can accept protons. This definition is rooted in the Brønsted-Lowry theory, which emphasizes the transfer of protons in acid-base reactions. Understanding this distinction is crucial for classifying compounds like H₂SO₃.
Recommended video:
Arrhenius Acids & Bases Concept 1
Sulfurous Acid (H₂SO₃)
H₂SO₃, or sulfurous acid, is a weak acid formed when sulfur dioxide (SO₂) dissolves in water. It can dissociate in solution to release H⁺ ions, which is characteristic of acids. Recognizing H₂SO₃ as an acid is essential for understanding its behavior in chemical reactions and its role in various environmental processes.
Recommended video:
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 (strongly acidic) to 14 (strongly basic), with 7 being neutral. Acids have a pH less than 7, while bases have a pH greater than 7. Knowing how to interpret pH values helps in identifying substances like H₂SO₃ as acidic based on their behavior in aqueous solutions.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution
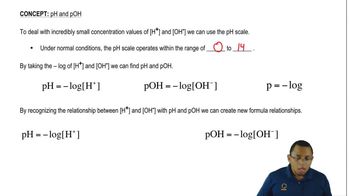

 0:40m
0:40m