Here are the essential concepts you must grasp in order to answer the question correctly.
Amino Acid Structure
Amino acids are organic compounds that serve as the building blocks of proteins. Each amino acid consists of a central carbon atom (the alpha carbon) bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a variable side chain (R group) that determines the specific properties of the amino acid.
Recommended video:
Amino Acid Catabolism: Amino Group Example 2
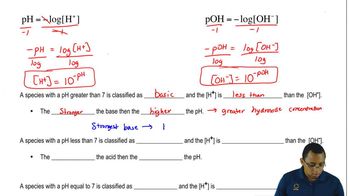
Physiological pH
Physiological pH refers to the typical pH level of human body fluids, which is around 7.4. At this pH, amino acids can exist in their zwitterionic form, where the amino group is protonated (-NH3+) and the carboxyl group is deprotonated (-COO-), affecting their charge and solubility.
Recommended video:
Lysine Properties
Lysine is a basic amino acid characterized by its side chain containing an additional amino group (-NH2). At physiological pH, lysine carries a positive charge due to the protonation of its side chain amino group, which influences its role in protein structure and function, particularly in binding to negatively charged molecules.
Recommended video:
Chemical Properties Example 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 5:16m
5:16m