Here are the essential concepts you must grasp in order to answer the question correctly.
Group 2A Elements
Group 2A, also known as the alkaline earth metals, includes elements like beryllium, magnesium, calcium, strontium, barium, and radium. These elements are characterized by having two electrons in their outermost shell, which they readily lose to form cations with a +2 charge. Understanding the properties of these elements is crucial for determining their metallic character.
Recommended video:
Periodic Table: Main Group Element Charges Concept 2
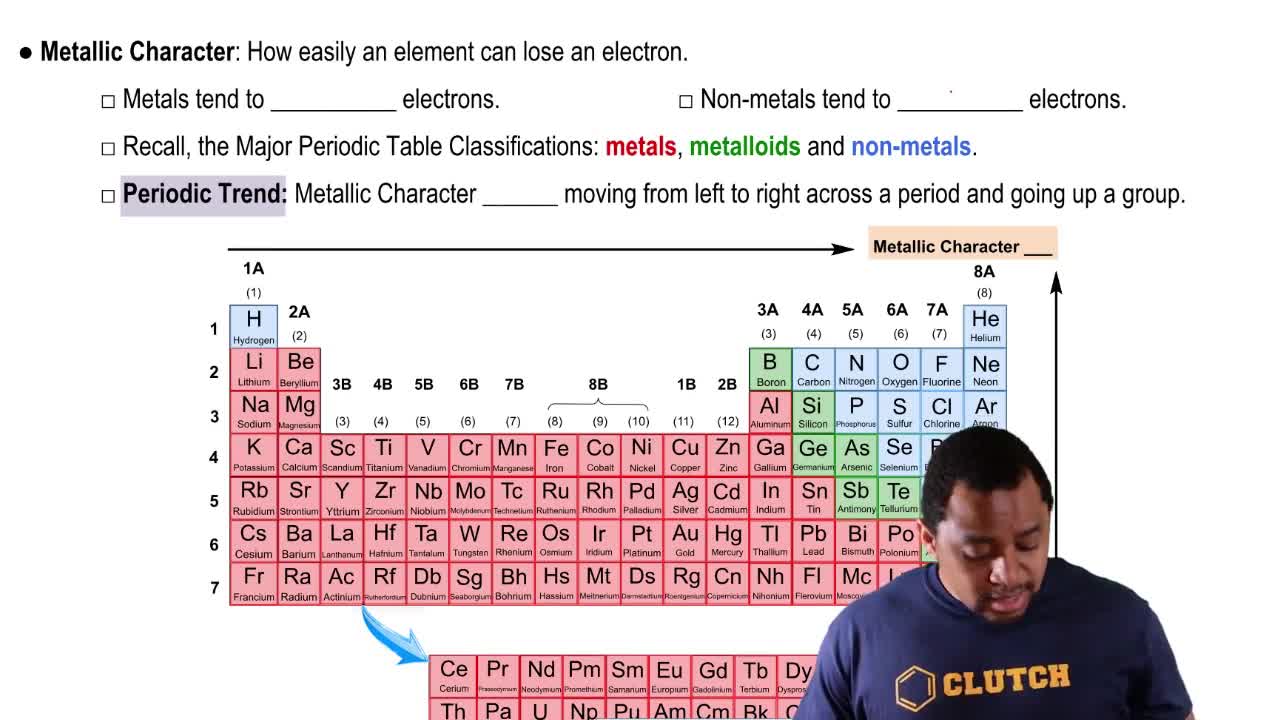
Metallic Character
Metallic character refers to the tendency of an element to lose electrons and form positive ions. It increases down a group in the periodic table due to the increasing atomic size and decreasing ionization energy. In Group 2A, this means that as you move from beryllium to radium, the elements become more metallic in nature.
Recommended video:
Periodic Trend: Metallic Character
Periodic Trends
Periodic trends are patterns observed in the periodic table that describe how certain properties of elements change across periods and down groups. For example, metallic character increases down a group, while electronegativity and ionization energy typically decrease. Recognizing these trends is essential for predicting the behavior of elements, including which has the most metallic character in Group 2A.
Recommended video:
Periodic Trend: Metallic Character
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 0:38m
0:38m