Here are the essential concepts you must grasp in order to answer the question correctly.
Hydronium Ion Concentration
The hydronium ion concentration, represented as [H₃O⁺], indicates the amount of hydronium ions present in a solution. It is a crucial factor in determining the acidity or basicity of a solution. A higher concentration of hydronium ions corresponds to a lower pH, indicating an acidic solution, while a lower concentration suggests a basic solution.
Recommended video:
Percent Concentrations Concept 1
pH Scale
The pH scale quantifies the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is considered neutral, while values below 7 indicate acidity and values above 7 indicate basicity. The pH is calculated using the formula pH = -log[H₃O⁺], making it essential to understand how hydronium ion concentration affects pH.
Recommended video:
Hydroxide Ion Concentration
The hydroxide ion concentration, denoted as [OH⁻], is the measure of hydroxide ions in a solution. It is inversely related to hydronium ion concentration through the water dissociation constant (Kw = [H₃O⁺][OH⁻] = 1.0 x 10⁻¹⁴ at 25°C). Understanding this relationship helps determine whether a solution is acidic, neutral, or basic based on the balance of these ions.
Recommended video:
Percent Concentrations Concept 1

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution
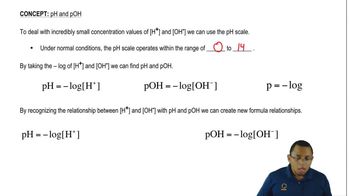


 1:31m
1:31m