Here are the essential concepts you must grasp in order to answer the question correctly.
Reactants and Products
In a chemical reaction, reactants are the starting substances that undergo a change, while products are the substances formed as a result of the reaction. In the given equation, sodium (Na) and water (H2O) are the reactants, and hydrogen gas (H2) and sodium hydroxide (NaOH) are the products.
Recommended video:
Solubility Product Constant (Ksp) Concept 2
Physical States
Physical states refer to the distinct forms that different phases of matter take on. In chemical equations, these states are indicated by symbols: (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous solution. Understanding these states helps in visualizing the reaction and the conditions under which it occurs.
Recommended video:
States of Matter Example 1
Chemical Equation
A chemical equation is a symbolic representation of a chemical reaction, showing the reactants on the left and the products on the right, separated by an arrow. It conveys not only the substances involved but also their physical states and the stoichiometry of the reaction, which is essential for understanding the quantities of reactants and products.
Recommended video:
Balancing Chemical Equations (Simplified) Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution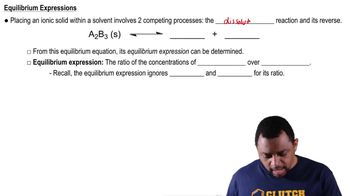



 0:58m
0:58m