Here are the essential concepts you must grasp in order to answer the question correctly.
Weak Electrolytes
Weak electrolytes are substances that partially dissociate into ions when dissolved in water. Unlike strong electrolytes, which completely ionize, weak electrolytes exist in equilibrium between their molecular form and their ionic form. This means that in a solution of a weak electrolyte, such as acetic acid, there will be a mixture of both ions and un-ionized molecules.
Recommended video:
Electrolytes (Simplified) Concept 2
Dissociation in Aqueous Solutions
Dissociation refers to the process by which a compound separates into its constituent ions in a solvent, typically water. In the case of acetic acid, HC₂H₃O₂, it dissociates into acetate ions (C₂H₃O₂⁻) and hydrogen ions (H⁺) to a limited extent. The degree of dissociation is crucial for determining the composition of the solution, as it influences whether the solution contains mostly molecules or ions.
Recommended video:
Equilibrium in Chemical Reactions
Equilibrium in chemical reactions occurs when the rate of the forward reaction equals the rate of the reverse reaction, resulting in constant concentrations of reactants and products. For weak electrolytes like acetic acid, this means that there is a dynamic balance between the dissociated ions and the undissociated molecules. Understanding this concept helps in predicting the behavior of weak acids in solution, including their ionization and the resulting composition.
Recommended video:
Chemical Equilibrium Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution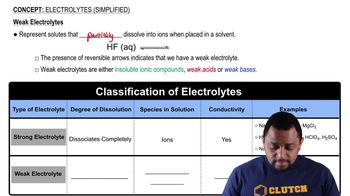



 2:38m
2:38m