Here are the essential concepts you must grasp in order to answer the question correctly.
Gas Solubility
Gas solubility refers to the ability of a gas to dissolve in a liquid, which is influenced by factors such as temperature, pressure, and the nature of the gas and solvent. At higher pressures, more gas molecules can enter the liquid phase, increasing solubility. Understanding the solubility of gases like O₂, N₂, CO, and CO₂ in water is crucial for applications in medicine and environmental science.
Recommended video:
Henry's Law
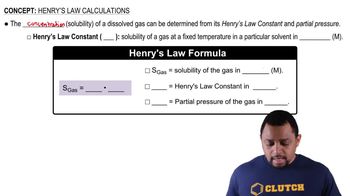
Henry's Law states that the amount of gas that dissolves in a liquid at a given temperature is directly proportional to the partial pressure of that gas above the liquid. This principle helps explain why gases like O₂ and CO₂ have different solubility levels in water under varying pressures, which is particularly relevant in hyperbaric treatments where pressure is significantly increased.
Recommended video:
Henry's Law Calculations Concept 1
Molecular Properties of Gases
The solubility of gases in water is also influenced by their molecular properties, such as polarity and molecular weight. For instance, carbon dioxide (CO₂) is more soluble than nitrogen (N₂) due to its ability to form weak interactions with water molecules. Understanding these properties helps predict how different gases behave in aqueous environments, which is essential for interpreting solubility trends.
Recommended video:
Chemical Properties Concept