Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation and Reduction Reactions
Oxidation and reduction (redox) reactions are chemical processes that involve the transfer of electrons between substances. Oxidation refers to the loss of electrons or an increase in oxidation state, while reduction involves the gain of electrons or a decrease in oxidation state. Understanding these definitions is crucial for identifying whether a reaction is an oxidation or reduction.
Recommended video:
Reduction Reactions Concept 1
Organic Functional Groups
Organic compounds are characterized by functional groups, which are specific groups of atoms that determine the chemical properties and reactions of the molecules. Recognizing these functional groups is essential for predicting how a compound will behave in a reaction, including whether it will undergo oxidation or reduction.
Recommended video:
Functional Group Priorities Concept 1
Electron Transfer Mechanisms
In organic chemistry, the mechanisms of electron transfer during oxidation and reduction reactions can vary significantly. Common mechanisms include the transfer of hydrogen atoms (which can be seen as a proxy for electron transfer) or the involvement of specific reagents that facilitate these changes. Understanding these mechanisms helps in accurately classifying the reactions.
Recommended video:
Electronic Structure: Electron Spin Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution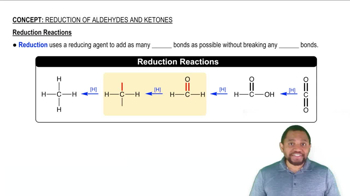


 3:12m
3:12m