Here are the essential concepts you must grasp in order to answer the question correctly.
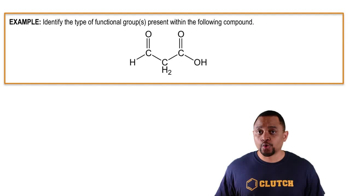
Carbonyl Group
The carbonyl group is a functional group characterized by a carbon atom double-bonded to an oxygen atom (C=O). It is a key feature in various organic compounds, including aldehydes and ketones. Understanding the structure and reactivity of the carbonyl group is essential for grasping how it interacts with nucleophiles, such as hydride ions, during reduction reactions.
Recommended video:
Functional Groups with Carbonyls Example 3
Hydride Ion (H⁻)
A hydride ion is a negatively charged ion (anion) consisting of one hydrogen atom with an extra electron. In organic chemistry, hydride ions act as strong nucleophiles, meaning they can donate electrons to electrophilic centers, such as the carbon atom in a carbonyl group. This donation leads to the reduction of the carbonyl compound, converting it into an alcohol.
Recommended video:
Proton (H⁺)
A proton, denoted as H⁺, is a positively charged hydrogen ion that plays a crucial role in acid-base chemistry and various biochemical processes. In the context of carbonyl reduction, protons are often involved in subsequent steps after the addition of a hydride ion, helping to stabilize the resulting intermediate and facilitating the conversion of the carbonyl compound into an alcohol.
Recommended video:
Acid and Base Strength Concept 4