Here are the essential concepts you must grasp in order to answer the question correctly.
Enzyme-Substrate Interaction
Enzymes are biological catalysts that speed up chemical reactions by binding to substrates at their active sites. The interaction is primarily driven by non-covalent forces such as hydrogen bonds, ionic interactions, and hydrophobic effects, which stabilize the enzyme-substrate complex. Understanding this interaction is crucial for determining which amino acids can effectively participate in binding.
Recommended video:
Enzyme-Substrate Complex Concept 1
Amino Acid Properties
Amino acids have distinct side chains that determine their chemical properties, such as polarity, charge, and size. For instance, serine has a polar side chain, lysine is positively charged at physiological pH, and glutamate carries a negative charge. These properties influence their ability to interact with substrates in the active site, making it essential to consider them when evaluating potential interactions.
Recommended video:
Amino Acid Catabolism: Amino Group Example 2
Active Site Specificity
The active site of an enzyme is specifically shaped to bind certain substrates, often described by the 'lock and key' or 'induced fit' models. This specificity means that only substrates with complementary shapes and chemical properties can effectively bind. Evaluating whether serine, lysine, or glutamate could be present in the active site requires analyzing how their properties align with the substrate's characteristics.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution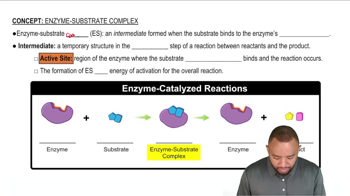



 2:32m
2:32m