Here are the essential concepts you must grasp in order to answer the question correctly.
Concentration
Concentration refers to the amount of solute present in a given volume of solution. In this case, a 10.0% (v/v) acetic acid solution means that there are 10 milliliters of acetic acid in every 100 milliliters of solution. Understanding concentration is crucial for calculating the required volume or mass of solute needed to achieve a specific solution concentration.
Recommended video:
Percent Concentrations Concept 1
Volume and Volume Percent
Volume percent (v/v) is a way to express the concentration of a solution, indicating the volume of solute divided by the total volume of the solution, multiplied by 100. For a 10.0% (v/v) acetic acid solution, this means that 10 mL of acetic acid is present in 100 mL of the final solution. This concept is essential for determining how much acetic acid is needed for a specific volume of solution.
Recommended video:
Percent Concentrations Concept 1
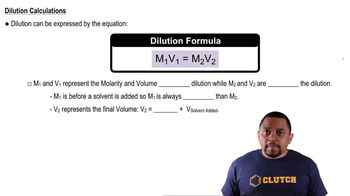
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. In preparing a solution, understanding how to dilute a concentrated solution to achieve the desired concentration is vital. This concept helps in calculating the necessary amounts of solute and solvent to create the target solution concentration.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 0:52m
0:52m