Here are the essential concepts you must grasp in order to answer the question correctly.
Acids and Bases
Acids and bases are fundamental concepts in chemistry that describe substances based on their ability to donate or accept protons (H+ ions). Acids are proton donors, while bases are proton acceptors. This classification is essential for understanding chemical reactions, pH levels, and the behavior of various compounds in solution.
Recommended video:
Arrhenius Acids & Bases Concept 1
Strong Acids
Strong acids are substances that completely dissociate in water, releasing all of their protons into the solution. This results in a high concentration of H+ ions, leading to a low pH. Hydroiodic acid (HI) is classified as a strong acid because it fully ionizes in aqueous solution, making it a key example in acid-base chemistry.
Recommended video:
pH Scale
The pH scale is a logarithmic scale used to measure the acidity or basicity of a solution, ranging from 0 to 14. A pH less than 7 indicates an acidic solution, while a pH greater than 7 indicates a basic solution. Understanding the pH scale is crucial for determining the strength of acids and bases, as well as their effects in various chemical contexts.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

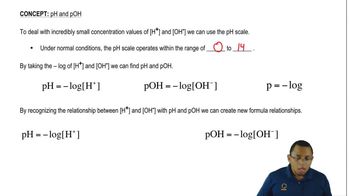

 0:40m
0:40m