Here are the essential concepts you must grasp in order to answer the question correctly.
Tertiary Structure of Proteins
The tertiary structure refers to the three-dimensional shape of a protein, formed by the folding of its polypeptide chain. This structure is stabilized by various interactions, including hydrogen bonds, ionic bonds, hydrophobic interactions, and disulfide bridges. Understanding the tertiary structure is crucial for predicting how a protein will function and interact with other molecules.
Recommended video:
Tertiary Protein Structure Concept 1
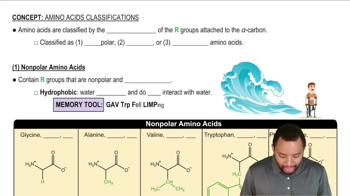
Polar and Nonpolar Amino Acids
Amino acids can be classified based on the properties of their R groups (side chains) as polar or nonpolar. Nonpolar R groups tend to be hydrophobic and are usually found in the interior of proteins, away from the aqueous environment, while polar R groups are hydrophilic and often located on the exterior, interacting with water. This distribution is essential for the protein's stability and function.
Recommended video:
Nonpolar Amino Acids Concept 1
Hydrophobic Effect
The hydrophobic effect is a key driving force in protein folding, where nonpolar amino acids aggregate to minimize their exposure to water. This phenomenon leads to the formation of a stable core in the protein structure, while polar amino acids are positioned on the surface, allowing them to interact with the aqueous environment. Understanding this effect is vital for predicting the arrangement of amino acids in the tertiary structure.
Recommended video:
Solubility: Temperature Effect Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:7m
2:7m