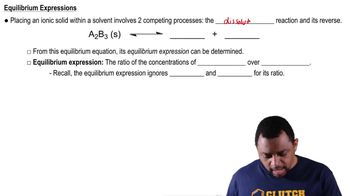
Here are the essential concepts you must grasp in order to answer the question correctly.
Ion Product of Water (K_w)
K_w, or the ion product of water, is a constant that represents the equilibrium constant for the self-ionization of water. It is defined as the product of the concentrations of hydrogen ions [H⁺] and hydroxide ions [OH⁻] in pure water at a given temperature. This concept is crucial for understanding acid-base chemistry and the behavior of water in various chemical reactions.
Recommended video:
Solubility Product Constant (Ksp) Concept 2
Self-Ionization of Water
The self-ionization of water is a process where two water molecules interact to produce one hydronium ion (H₃O⁺) and one hydroxide ion (OH⁻). This reaction is reversible and occurs to a very small extent in pure water, leading to the formation of equal concentrations of H⁺ and OH⁻ ions. Understanding this process is essential for grasping the concept of K_w and its implications in acid-base equilibria.
Recommended video:
Numerical Value of K_w at 25 °C
At 25 °C, the numerical value of K_w is 1.0 x 10⁻¹⁴. This value indicates that in pure water, the concentrations of H⁺ and OH⁻ ions are both 1.0 x 10⁻⁷ M, reflecting the neutral nature of pure water at this temperature. This constant is fundamental in calculating pH and understanding the acidity or basicity of solutions.
Recommended video:
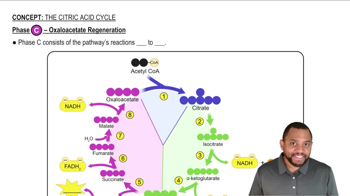
Phase C - Oxaloacetate Regeneration Concept 7