Here are the essential concepts you must grasp in order to answer the question correctly.
Acids
Acids are substances that can donate protons (H⁺ ions) in a chemical reaction. They typically have a sour taste and can turn blue litmus paper red. Common examples include hydrochloric acid (HCl) and sulfuric acid (H₂SO₄). Acids are characterized by their ability to increase the concentration of hydrogen ions in a solution.
Recommended video:
Bases
Bases are substances that can accept protons or donate hydroxide ions (OH⁻) in a chemical reaction. They usually have a bitter taste and can turn red litmus paper blue. Examples include sodium hydroxide (NaOH) and ammonia (NH₃). Bases increase the concentration of hydroxide ions in a solution, which can neutralize acids.
Recommended video:
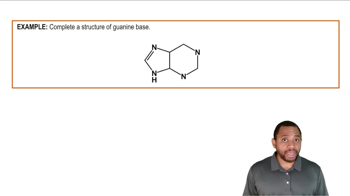
Nitrogenous Bases Example 3
pH Scale
The pH scale is a measure of the acidity or basicity of a solution, ranging from 0 to 14. A pH less than 7 indicates an acidic solution, while a pH greater than 7 indicates a basic solution. A pH of 7 is considered neutral, as seen in pure water. The scale is logarithmic, meaning each whole number change represents a tenfold change in acidity or basicity.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution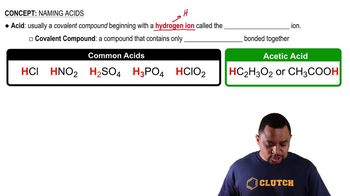
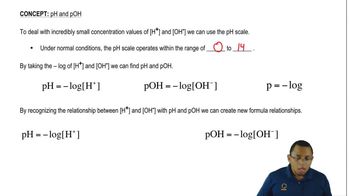

 0:40m
0:40m