Here are the essential concepts you must grasp in order to answer the question correctly.
pH and pOH Relationship
The pH and pOH of a solution are related through the equation pH + pOH = 14 at 25°C. This relationship allows us to calculate one from the other. Since pH measures the concentration of hydrogen ions [H₃O⁺] and pOH measures the concentration of hydroxide ions [OH⁻], knowing one enables the determination of the other.
Recommended video:
Ion Product of Water
The ion product of water (Kw) is a constant at a given temperature, defined as Kw = [H₃O⁺][OH⁻]. At 25°C, Kw is 1.0 x 10⁻¹⁴. This relationship is crucial for calculating the concentration of hydronium ions when the concentration of hydroxide ions is known, as it allows for the determination of [H₃O⁺] from [OH⁻].
Recommended video:
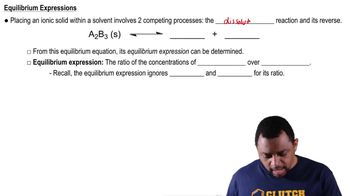
Solubility Product Constant (Ksp) Concept 2
Concentration Units in Chemistry
Concentration in chemistry is often expressed in molarity (M), which is moles of solute per liter of solution. Understanding how to manipulate these units is essential for calculations involving [H₃O⁺] and [OH⁻]. In this context, knowing the molarity of hydroxide ions allows for the calculation of hydronium ion concentration using the relationships defined by pH, pOH, and Kw.
Recommended video:
Percent Concentrations Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:31m
1:31m