Here are the essential concepts you must grasp in order to answer the question correctly.
Hemiacetals and Hemiketals
Hemiacetals and hemiketals are organic compounds formed when an alcohol reacts with an aldehyde or ketone, respectively. In this reaction, one molecule of alcohol adds to the carbonyl carbon, resulting in a structure that contains both an alcohol and an ether functional group. Hemiacetals and hemiketals are typically unstable and can further react to form acetals or ketals when a second alcohol molecule is added.
Recommended video:
Cyclic Hemiacetals Concept 1
Acetals and Ketals
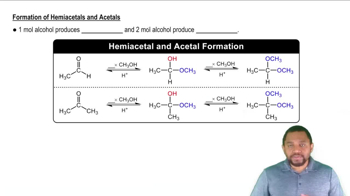
Acetals and ketals are derived from hemiacetals and hemiketals through a reaction with an additional alcohol molecule, leading to the formation of a stable compound. Acetals are formed from aldehydes, while ketals are formed from ketones. These compounds are characterized by having two alkoxy groups attached to the same carbon atom, making them resistant to hydrolysis under neutral conditions but susceptible to hydrolysis in acidic environments.
Recommended video:
Formation of Hemiacetals and Acetals Concept 2
Hydrolysis of Acetals and Ketals
Hydrolysis of acetals and ketals involves the reaction with water, typically in the presence of an acid catalyst, to revert them back to their original carbonyl compounds and alcohols. This process is essential in organic chemistry as it demonstrates the reversibility of acetal and ketal formation. The complete hydrolysis products of acetals and ketals are the corresponding aldehydes or ketones and the alcohols used in their formation.
Recommended video:
Formation of Hemiacetals and Acetals Concept 2
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution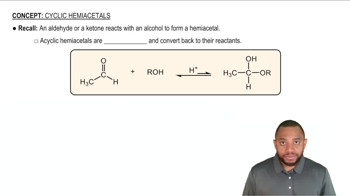


 2:38m
2:38m