Here are the essential concepts you must grasp in order to answer the question correctly.
Exothermic Reaction
An exothermic reaction is a chemical process that releases energy, usually in the form of heat, to its surroundings. This occurs when the total energy of the products is lower than that of the reactants, resulting in a net release of energy. Common examples include combustion reactions, where fuels react with oxygen to produce heat and light.
Recommended video:
Endothermic & Exothermic Reactions
Energy Diagram
An energy diagram is a graphical representation that illustrates the energy changes during a chemical reaction. It typically shows the energy of the reactants and products, as well as the activation energy required to initiate the reaction. For exothermic reactions, the diagram will depict a downward slope from reactants to products, indicating energy release.
Recommended video:
Energy Diagrams Example 1
Activation Energy
Activation energy is the minimum energy required for a chemical reaction to occur. It represents the energy barrier that reactants must overcome to transform into products. In an energy diagram, this is shown as the peak of the curve, where the energy is at its highest before the reaction proceeds to form products, which are at a lower energy level in exothermic reactions.
Recommended video:
Factors Affecting Enzyme Activity Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution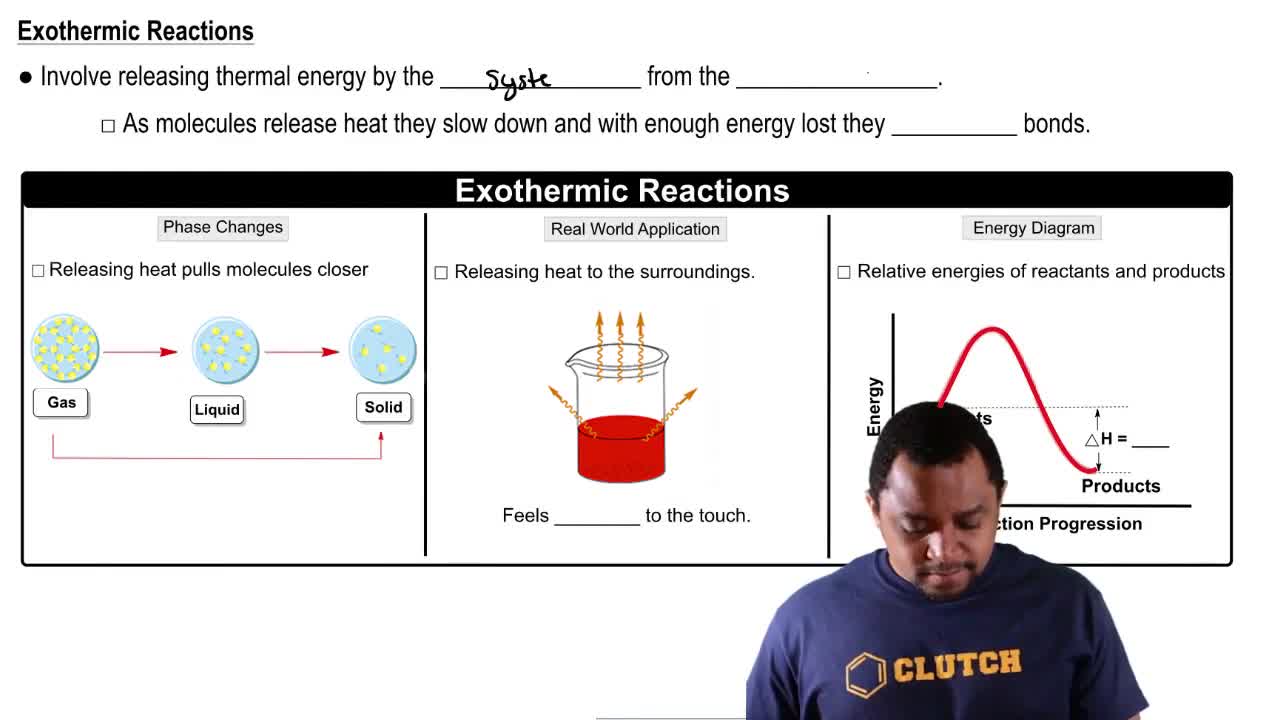



 2:40m
2:40m