Here are the essential concepts you must grasp in order to answer the question correctly.
Covalent Bonds
Covalent bonds are formed when two atoms share one or more pairs of electrons, resulting in a stable balance of attractive and repulsive forces between the atoms. This type of bond typically occurs between nonmetals and is characterized by the strength and stability it provides to molecules. An example is the bond between hydrogen and oxygen in water (H2O).
Recommended video:
Noncovalent Interactions
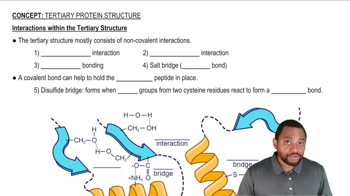
Noncovalent interactions are weaker than covalent bonds and do not involve the sharing of electrons. These interactions include hydrogen bonds, ionic bonds, van der Waals forces, and hydrophobic interactions. They play crucial roles in biological processes, such as protein folding and enzyme-substrate binding, where transient interactions are essential for function.
Recommended video:
Interactions within the Tertiary Structure Concept 2
Chemical Context
Understanding the chemical context of a problem involves recognizing the types of atoms involved, their electronegativities, and the molecular structure. This context helps determine whether the interactions between pairs of atoms are likely to be covalent or noncovalent. For example, the presence of highly electronegative atoms like oxygen or nitrogen can influence the nature of the bonds formed.
Recommended video:
Chemical Reaction: Chemical Change Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:7m
2:7m