Here are the essential concepts you must grasp in order to answer the question correctly.
Acids
Acids are substances that can donate protons (H+) in a chemical reaction. They typically have a sour taste, which is a common characteristic of many acidic substances, such as citric acid found in lemons. Acids can also turn blue litmus paper red and react with bases to form salts and water.
Recommended video:
Bases
Bases are substances that can accept protons or donate hydroxide ions (OH-) in a chemical reaction. They often have a bitter taste and a slippery feel. Bases can turn red litmus paper blue and react with acids to neutralize them, forming salts and water.
Recommended video:
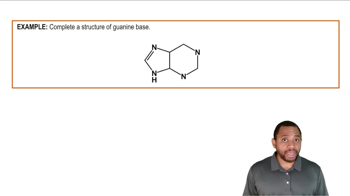
Nitrogenous Bases Example 3
pH Scale
The pH scale is a measure of the acidity or basicity of a solution, ranging from 0 to 14. A pH less than 7 indicates an acidic solution, while a pH greater than 7 indicates a basic solution. The pH scale helps in understanding the strength of acids and bases, with strong acids having lower pH values and strong bases having higher pH values.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution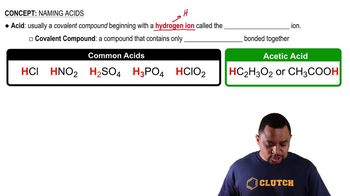
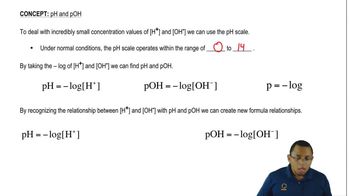

 0:40m
0:40m