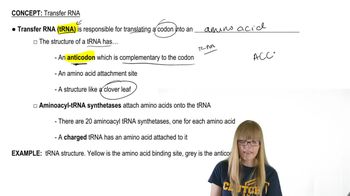Table of contents
- 1. Introduction to Genetics51m
- 2. Mendel's Laws of Inheritance3h 37m
- 3. Extensions to Mendelian Inheritance2h 41m
- 4. Genetic Mapping and Linkage2h 28m
- 5. Genetics of Bacteria and Viruses1h 21m
- 6. Chromosomal Variation1h 48m
- 7. DNA and Chromosome Structure56m
- 8. DNA Replication1h 10m
- 9. Mitosis and Meiosis1h 34m
- 10. Transcription1h 0m
- 11. Translation58m
- 12. Gene Regulation in Prokaryotes1h 19m
- 13. Gene Regulation in Eukaryotes44m
- 14. Genetic Control of Development44m
- 15. Genomes and Genomics1h 50m
- 16. Transposable Elements47m
- 17. Mutation, Repair, and Recombination1h 6m
- 18. Molecular Genetic Tools19m
- 19. Cancer Genetics29m
- 20. Quantitative Genetics1h 26m
- 21. Population Genetics50m
- 22. Evolutionary Genetics29m
12. Gene Regulation in Prokaryotes
Tryptophan Operon and Attenuation
Problem 32b
Textbook Question
Section 9.4 describes the function of tRNA synthetases in attaching amino acids to tRNAs (see Figure 9.16). Suppose the tRNA synthetase responsible for attaching tryptophan to tRNA is mutated in a bacterial strain with the result that the tRNA synthetase functions at about 15% of the efficiency of the wild-type tRNA synthetase. How would this mutation affect attenuation of the tryptophan operon? Explain your answer.
 Verified step by step guidance
Verified step by step guidance1
Understand the role of tRNA synthetase: tRNA synthetases are enzymes that attach specific amino acids to their corresponding tRNAs. In this case, the enzyme attaches tryptophan to its tRNA.
Recognize the impact of reduced efficiency: A mutation causing the tRNA synthetase to function at 15% efficiency means that tryptophan is attached to tRNA at a much slower rate.
Consider the tryptophan operon: The tryptophan operon is a group of genes used to synthesize tryptophan. It is regulated by attenuation, which is a mechanism that controls gene expression based on tryptophan levels.
Analyze the effect on attenuation: With reduced efficiency of tRNA synthetase, there will be less charged tRNA^Trp available, potentially leading to a perceived low tryptophan level in the cell.
Predict the outcome: The low level of charged tRNA^Trp may cause the ribosome to stall at the leader peptide region of the mRNA, preventing the formation of the terminator structure and allowing transcription of the tryptophan operon to continue.
Recommended similar problem, with video answer:
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
tRNA Synthetases
tRNA synthetases are enzymes that catalyze the attachment of specific amino acids to their corresponding transfer RNA (tRNA) molecules. This process, known as aminoacylation, is crucial for accurate protein synthesis, as it ensures that the correct amino acid is incorporated into the growing polypeptide chain during translation. A mutation that reduces the efficiency of a tRNA synthetase can lead to a decrease in the availability of the corresponding amino acid for protein synthesis.
Recommended video:
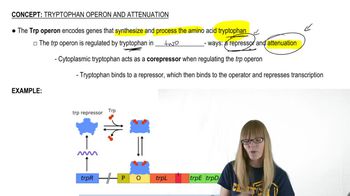
Attenuation in the Tryptophan Operon
Attenuation is a regulatory mechanism in bacterial operons, particularly the tryptophan operon, that controls gene expression based on the availability of tryptophan. It involves the formation of specific RNA structures during transcription that can either promote or terminate the synthesis of mRNA, depending on the levels of tryptophan. When tryptophan is abundant, the operon is repressed; when it is scarce, transcription proceeds, allowing for the production of enzymes needed for tryptophan synthesis.
Recommended video:
Guided course

Trp Attenuation
Impact of Mutation on Gene Regulation
A mutation in the tRNA synthetase that reduces its efficiency affects the overall availability of tryptophan for protein synthesis. If tryptophan levels drop due to inefficient amino acid attachment, the attenuation mechanism may be altered, potentially leading to increased expression of the tryptophan operon. This change can disrupt the balance of tryptophan synthesis and utilization, impacting the cell's ability to respond to its metabolic needs effectively.
Recommended video:
Guided course

Review of Regulation