Arrange these elements in order of increasing first ionization energy: Si, F, In, N.
Choose the element with the more negative (more exothermic) electron affinity from each pair. b. C or F
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Electron Affinity

Trends in Electron Affinity
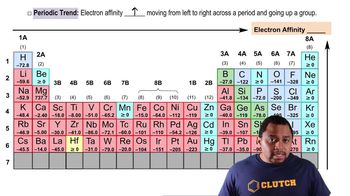
Comparison of Carbon and Fluorine

For each element, predict where the "jump" occurs for successive ionization energies. (For example, does the jump occur between the first and second ionization energies, the second and third, the third and fourth, and so on?) a. B b. Na C. P d. S
Consider this set of ionization energies.
IE1 = l000 kJ/mol
IE2 = 2250 kJ/mol
IE3 = 3360 kJ/mol
IE4 = 4560 kJ/mol
IE5 = 7010 kJ/mol
IE6 = 8500 kJ/mol
IE = 27,I00 kJ/mol
To which third-period element do these ionization values belong?
Choose the element with the more negative (more exothermic) electron affinity from each pair. c. P or S
Choose the element with the more negative (more exothermic) electron affinity in each pair. a. Ca or Se b. Ge or S c. Al or O d. Se or I
Choose the more metallic element from each pair. a. Rb or Sn b. P or Sb c. Te or Cl d. O or P
