Choose the element with the higher first ionization energy from each pair. a. Ge or Br b. P or In c. S or Te d. As or Br
Ch.9 - Periodic Properties of the Elements
Chapter 9, Problem 82
Consider this set of ionization energies.
IE1 = l000 kJ/mol
IE2 = 2250 kJ/mol
IE3 = 3360 kJ/mol
IE4 = 4560 kJ/mol
IE5 = 7010 kJ/mol
IE6 = 8500 kJ/mol
IE = 27,I00 kJ/mol
To which third-period element do these ionization values belong?
 Verified step by step guidance
Verified step by step guidance1
Identify the trend in ionization energies: Ionization energy generally increases as more electrons are removed from an atom. This is because the remaining electrons experience a stronger effective nuclear charge as the electron-electron repulsion decreases.
Examine the significant jumps in ionization energies: Large increases in ionization energy values typically indicate the removal of an electron from a new, more stable electron shell or subshell. For example, a significant jump from IE5 to IE6 suggests that removing the sixth electron involves breaking into a more stable, closer shell to the nucleus.
Compare the ionization energy values with known values for third-period elements: By comparing the given ionization energies with standard values for elements in the third period (Sodium to Argon), you can identify which element these ionization energies most likely correspond to.
Consider electron configuration: The electron configuration of the element can help explain the pattern of ionization energies. For instance, elements with a half-filled or fully filled p-orbital will have higher ionization energies due to increased electron stability.
Use the periodic table as a reference: Locate the third-period elements on the periodic table and use their known properties and electron configurations to match the given ionization energy pattern to the correct element.

Verified Solution
Video duration:
0m:0sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ionization Energy
Ionization energy is the amount of energy required to remove an electron from an atom or ion in its gaseous state. It is a key indicator of an element's reactivity and can vary significantly among different elements. Generally, ionization energy increases across a period in the periodic table due to increasing nuclear charge, which holds electrons more tightly.
Recommended video:
Guided course

Ionization Energy
Trends in Ionization Energies
Ionization energies exhibit specific trends in the periodic table. As you move from left to right across a period, ionization energy typically increases due to the increased effective nuclear charge. Conversely, as you move down a group, ionization energy decreases because the outer electrons are further from the nucleus and experience greater shielding from inner electrons.
Recommended video:
Guided course
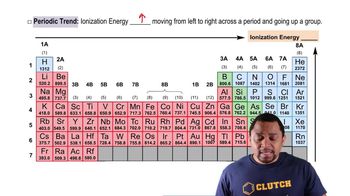
Ionization Energy Trends
Identification of Elements
To identify an element based on its ionization energies, one can analyze the pattern of the provided values. A significant jump in ionization energy values indicates the removal of an electron from a new, more stable electron shell, suggesting the element's group. In this case, the provided ionization energies suggest the element is likely to be a third-period element, such as phosphorus or sulfur, based on the increasing energy values.
Recommended video:
Guided course
Chalcogen Identification Example
Related Practice
Textbook Question
Textbook Question
Arrange these elements in order of increasing first ionization energy: Si, F, In, N.
1501
views
2
rank
Textbook Question
For each element, predict where the "jump" occurs for successive ionization energies. (For example, does the jump occur between the first and second ionization energies, the second and third, the third and fourth, and so on?) a. B b. Na C. P d. S
Textbook Question
Choose the element with the more negative (more exothermic) electron affinity from each pair. b. C or F
Textbook Question
Choose the element with the more negative (more exothermic) electron affinity from each pair. c. P or S
Textbook Question
Choose the element with the more negative (more exothermic) electron affinity in each pair. a. Ca or Se b. Ge or S c. Al or O d. Se or I
