Determine the molecular geometry and sketch each molecule or ion using the bond conventions shown in 'Representing Molecular Geometries on Paper' in Section 10.4. d. IBr4-
 Tro 5th Edition
Tro 5th Edition Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory
Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory Problem 40d
Problem 40dDetermine the molecular geometry and sketch each molecule or ion, using the bond conventions shown in 'Representing Molecular Geometries on Paper' in Section 10.4. d. IF4+
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
VSEPR Theory
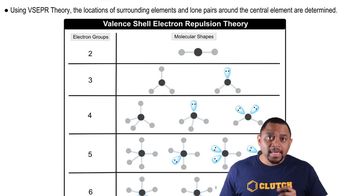
Molecular Geometry

Bonding and Lone Pairs
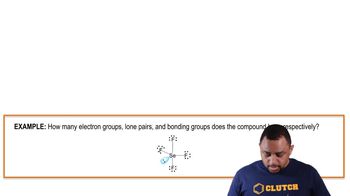
Determine the molecular geometry and sketch each molecule or ion, using the bond conventions shown in 'Representing Molecular Geometries on Paper' in Section 10.4. b. SCl6
Determine the molecular geometry and sketch each molecule or ion, using the bond conventions shown in 'Representing Molecular Geometries on Paper' in Section 10.4. c. PF5
Determine the molecular geometry about each interior atom and draw each molecule. (Skeletal structure is indicated in parentheses.)
a. C2H2 (skeletal structure HCCH)
b. C2H4 (skeletal structure H2CCH2)
c. C2H6 (skeletal structure H3CCH3)
Determine the molecular geometry about each interior atom and sketch each molecule. a. N2
Determine the molecular geometry about each interior atom and sketch each molecule. b. N2H2 (skeletal structure HNNH)