An ibuprofen suspension for infants contains 100 mg/5.0 mL suspension. The recommended dose is 10 mg/kg body weight. How many mL of this suspension should be given to an infant weighing 18 lb? (Assume two significant figures.)
Classify each property as intensive or extensive. a. volume b. boiling point c. temperature d. electrical conductivity e. energy
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
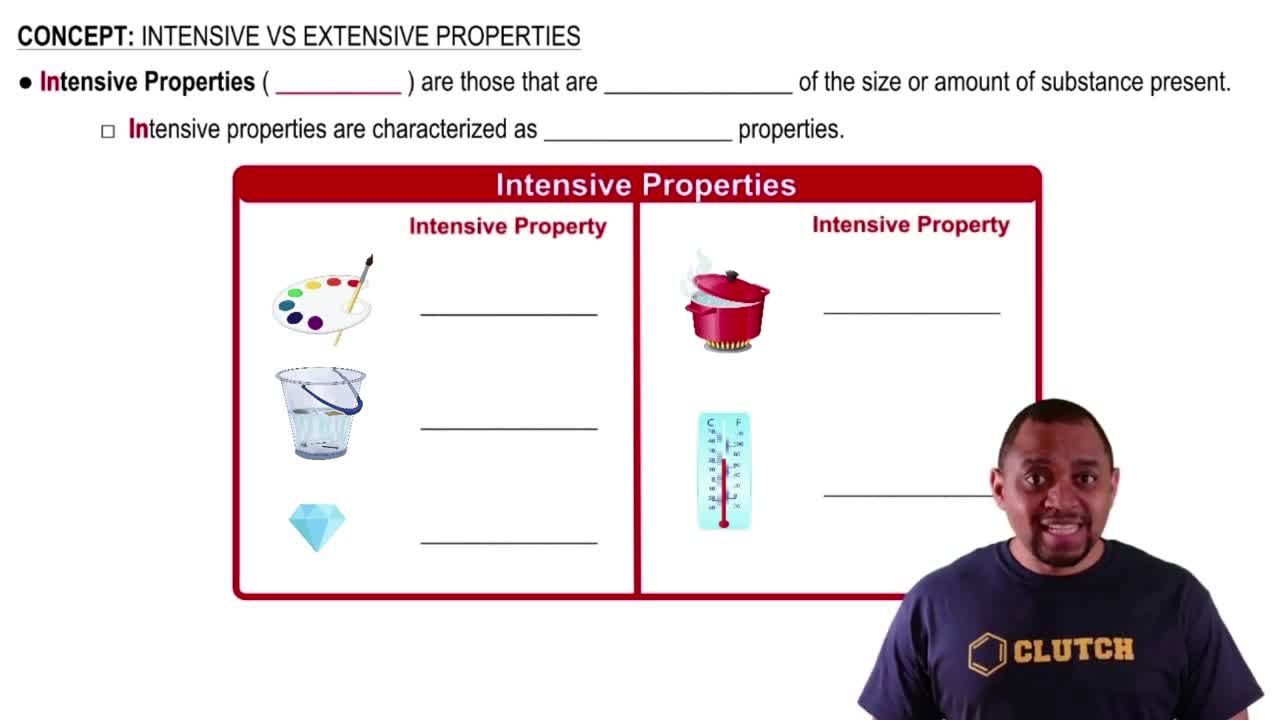
Intensive Properties
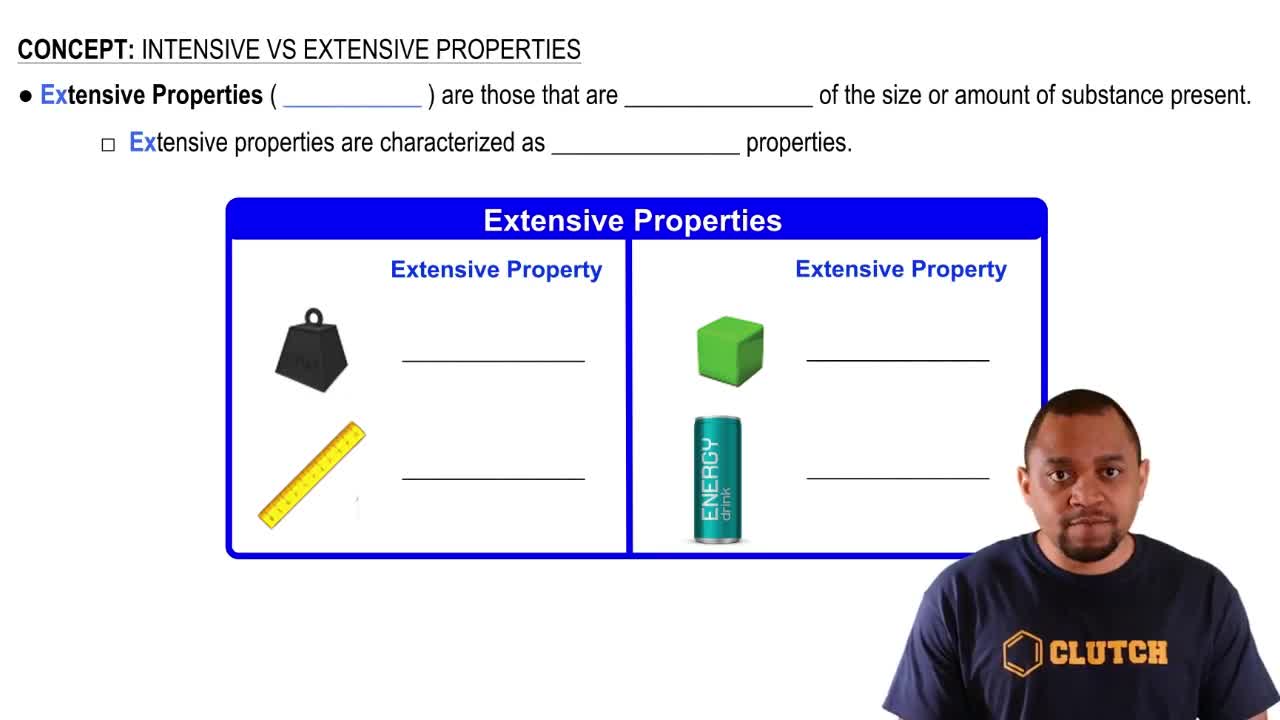
Extensive Properties
Classification of Properties

There are exactly 60 seconds in a minute, exactly 60 minutes in an hour, exactly 24 hours in a mean solar day, and 365.24 solar days in a solar year. How many seconds are in a solar year? Give your answer with the correct number of significant figures.
Determine the number of picoseconds in 2.0 hours.
At what temperatures are the readings on the Fahrenheit and Celsius thermometers the same?
On a new Jekyll temperature scale, water freezes at 17 °J and boils at 97 °J. On another new temperature scale, the Hyde scale, water freezes at 0 °H and boils at 120 °H. If methyl alcohol boils at 84 °H, what is its boiling point on the Jekyll scale?
Force is defined as mass times acceleration. Starting with SI base units, derive a unit for force. Using SI prefixes, suggest a convenient unit for the force resulting from a collision with a 10-ton trailer truck moving at 55 mi per hour and for the force resulting from the collision of a molecule of mass around 10 - 20 kg moving almost at the speed of light (3×108 m/s) with the wall of its container. (Assume a 1-second deceleration time for both collisions.)
