When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. c. Sketch a qualitative energy diagram similar to Figure 13.7 for the dissolution of LiI.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Dissolution Process

Exothermic Reactions
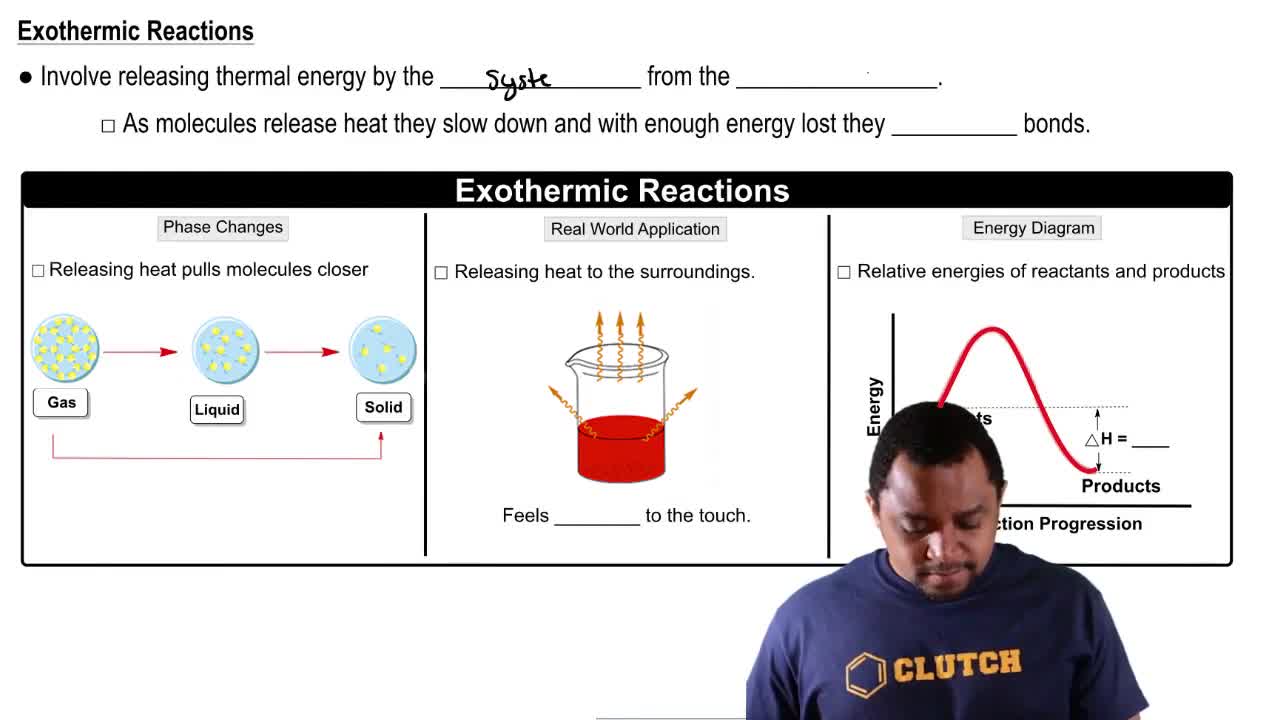
Energy Diagrams

When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. a. Is the dissolution of lithium iodide endothermic or exothermic?
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. b. What can you conclude about the relative magnitudes of the lattice energy of lithium iodide and its heat of hydration?
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. d. Why does the solution form? What drives the process?
Silver nitrate has a lattice energy of -820 kJ/mol and a heat of solution of 22.6 kJ/mol. Calculate the heat of hydration for silver nitrate.
Use the data to calculate the heats of hydration of lithium chloride and sodium chloride. Which of the two cations, lithium or sodium, has stronger ion–dipole interactions with water? Why?
