Construct a Born–Haber cycle for the formation of the hypothetical compound NaCl2, where the sodium ion has a 2+ charge (the second ionization energy for sodium is given in Table 7.2). (a) How large would the lattice energy need to be for the formation of NaCl2 to be exothermic?
Consider the collection of nonmetallic elements O, P, Te, I, and B. (b) Which two would form the longest single bond?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
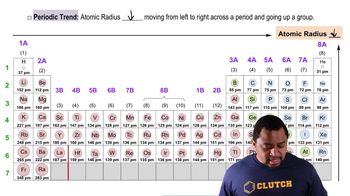
Bond Length

Atomic Size and Trends
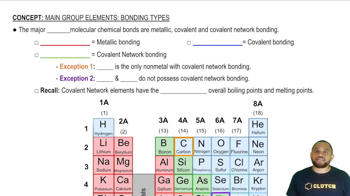
Types of Bonds
Consider the collection of nonmetallic elements O, P, Te, I, and B. (a) Which two would form the most polar single bond?
The substance chlorine monoxide, ClO(g), is important in atmospheric processes that lead to depletion of the ozone layer. The ClO molecule has an experimental dipole moment of 1.24 D, and the Cl — O bond length is 160 pm. (b) Based on the electronegativities of the elements, which atom would you expect to have a partial negative charge in the ClO molecule?
(b) Using these partial charges and the atomic radii given in Figure 7.8, estimate the dipole moment of the molecule.
(c) The measured dipole moment of BrCl is 0.57 D. If you assume the bond length in BrCl is the sum of the atomic radii, what are the partial charges on the atoms in BrCl using the experimental dipole moment?
